Ozone layer:
The majority of the UV light from the Sun is absorbed by the Earth’s stratosphere’s ozone layer, also known as the ozone shield. Compared to other areas of the atmosphere, it has a high concentration of ozone, yet it is still relatively low in comparison to other gases in the stratosphere.

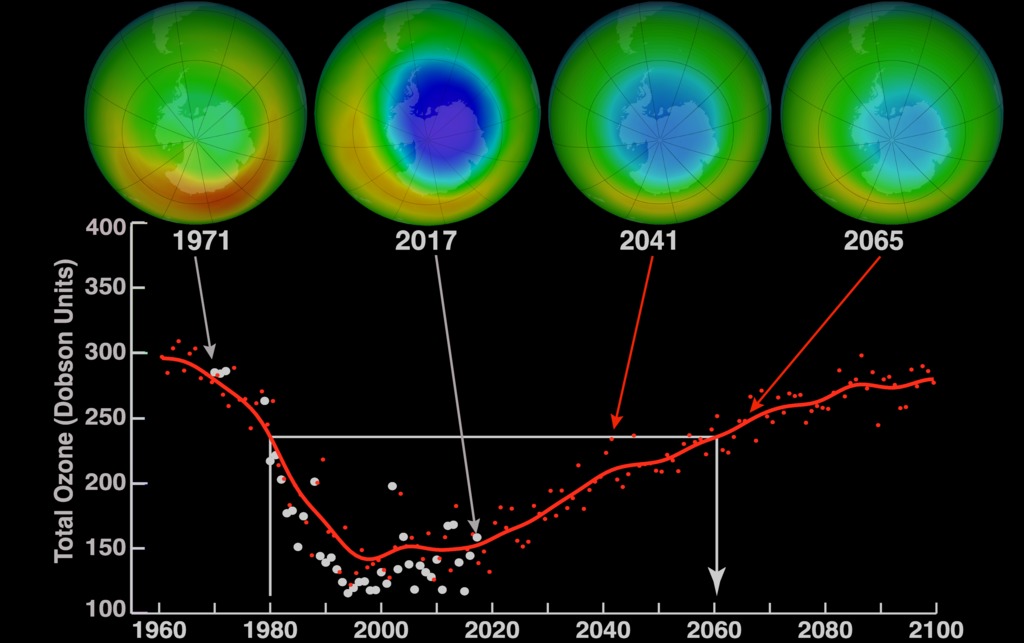
Ozone Layer Depletion:
The high atmosphere’s ozone layer gets thinned due to ozone layer depletion. This occurs when ozone molecules come into touch with chlorine and bromine atoms in the atmosphere and are broken down. Ozone molecules can be destroyed by one chlorine molecule. It doesn’t get made as quickly as it gets destroyed.

Effects of Ozone Layer Depletion:
The ozone layer blocks harmful ultraviolet (UVB) light wavelengths from entering the Earth’s atmosphere. These wavelengths hurt plants and animals as well as cause skin cancer, sunburn, permanent blindness, and cataracts, all of which were predicted to sharply increase as a result of the weakening of the ozone layer.
Increased UV radiation reaching Earth as a result of ozone depletion may increase the incidence of skin cancer, cataracts, and immune system impairment. The most lethal form of skin cancer, melanoma, is thought to be on the rise as a result of excessive UV exposure.
Causes of Ozone Layer Depletion:
Mainly there are 5 types of causes for Ozone Layer Depletion.
They are:
1.Chlorofluoro Carbons.
2.Nitrogenous Compounds
3.Bromine Compounds
4.Natural Causes
5.Fossil fuels destroy the Ozone Layer
1.Chlorofluoro Carbons:
CFCs, or chlorofluorocarbons, are to blame for the ozone layer’s thinning. The Ozone Layer helps block dangerous UV rays that would otherwise burn plants and cause skin cancer in people. Ozone molecules break down as a result of chemical processes brought on by CFCs, decreasing the amount of UV radiation that can be absorbed.
2.Nitrogenous Compounds:
Just above the region with the highest ozone concentrations, nitrogen oxides are the main cause of ozone depletion. As a result, NOx effectively destroys ozone . Because only around 10% of N2O is converted to NOx, compared to the CFCs’ potential contribution of all of their chlorine, the ODP of N2O is smaller than that of CFCs.
3.Bromine Compounds:
Loss of ozone. Ozone molecules are destroyed when chlorine and bromine atoms come into touch with them in the stratosphere. Until it is eliminated from the stratosphere, one chlorine atom can destroy more than 100,000 ozone molecules. More quickly than it is produced naturally, ozone can be destroyed.
4.Natural Causes:
It has been discovered that some natural processes, such solar flares and stratospheric winds, degrade the ozone layer. Yet, it only contributes to 1-2% of the ozone layer loss. The ozone layer is being destroyed due to volcanic eruptions as well.
5.Fossil Fuels destroy the Ozone:
The widespread usage of fossil fuels in daily life has brought about an era of ozone layer depletion and global warming. When burned, fossil fuels like gasoline, diesel, natural gas, etc. release dangerous greenhouse gases like CO, CO2, SO2, NOx, etc.
Effect of Ozone Layer Depletion on Environment:
ncreased UV-B rays that reach the earth’s surface as a result of stratospheric ozone loss have the potential to disrupt biological processes and harm a variety of materials. The common sunburn that results from excessive sun exposure is a good example of how UV -B can have an impact on biological processes.
Effect of Ozone Layer Depletion on Plants:
More dangerous UV rays are penetrating Earth’s surface as a result of the ozone layer being destroyed. As radiation levels rise, plants are unable to swiftly adapt, which can have a negative impact on their physiological and developmental processes.
Effect of Ozone Layer Depletion on Animals:
Early developmental stages of fish, shrimp, crab, amphibians, and other marine species have been discovered to be damaged by UVB light. Reduced fertility and hampered larval development are the most serious impacts.
Solutions for Ozone Layer Depletion:
1.Minimize the use of Vehicles
2.Use Eco-Friendly cleaning products
3.Pesticides should not be used
4.Ozone Depleting products Should not be used
5.Renewable sources of Energy.
6.Reuse and Recycle
1.Minimize the use of Vehicles:
The simplest method to stop ozone depletion is to minimise the number of cars on the road. Large amounts of greenhouse gases are released by these vehicles, which eventually condense into smog and contribute to the ozone layer’s thinning.
2.Use Eco-Friendly cleaning products:
The bulk of cleaning supplies for the home are made with harsh chemicals that leach into the air and thin the ozone layer. Use natural and environmentally friendly cleaning supplies to prevent this from happening.
3.Pesticides should not be used:
Pesticides are useful tools for managing weeds and pests on your farm, but they can dramatically harm the ozone layer. The most efficient technique to get rid of weeds and pests is with natural remedies. Simply hand-weed your farm and use environmentally safe pesticides as an option to combat pests.
4.Ozone Depleting products Should not be used:
When you go shopping, avoid purchasing products that contain chlorofluorocarbons in aerosol form. If the primary ingredient in your fire extinguishers is “halon” or “halogenated hydrocarbon,” check them out. Dispose of any outdated freezers and air conditioners that use chlorofluorocarbons. This could result in the discharge of toxic compounds into the atmosphere.
5.Renewable Sources of Energy:
In order to stop the destruction of the ozone layer, renewable energy sources must be used and developed. Fossil fuels like coal are a key source of electricity in addition to nuclear energy.
6.Reuse and Recycle:
We should also make an effort to reuse as many of our possessions as we can. We need to make sure they can be properly recycled if we stop using them. In this approach, we can lessen the demand on natural resources. As a result, we also prevent the harm that resource exploitation causes to the ecosystem.
.


You must be logged in to post a comment.